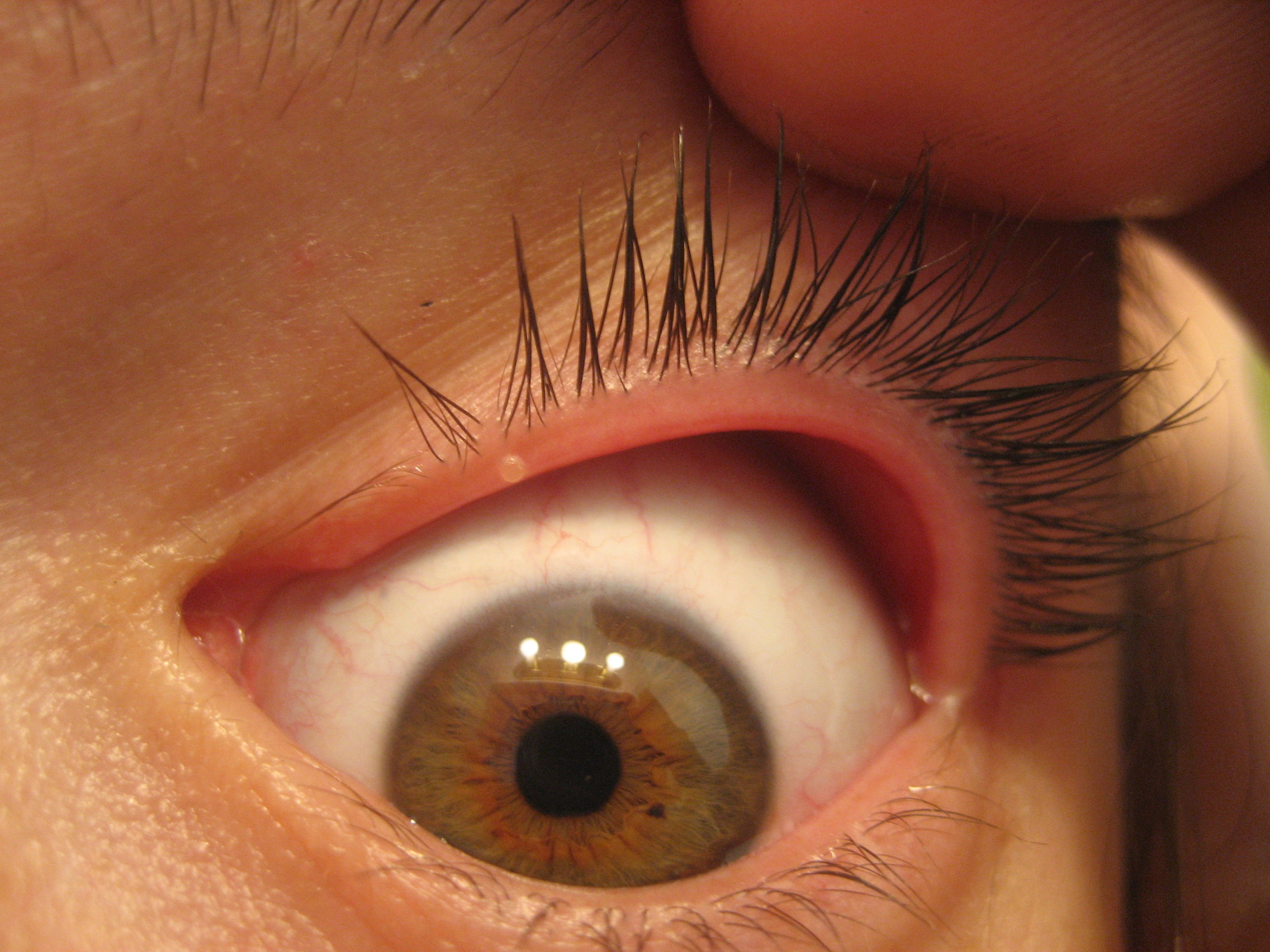
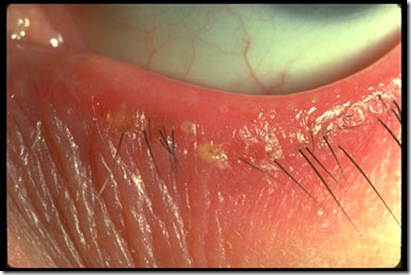
Abrasion is a defect in the surface of the cornea that is limited to the epithelial layers and that does not penetrate the Bowman membrane. In some cases, the bulbar conjunctiva is also involved. Corneal abrasion results from physical or chemical trauma. Severe corneal injuries can also involve the deeper, thicker stromal layer; in this situation, the term corneal ulcer may be used.
The conjunctival response to corneal wounding has been known since Mann first observed that peripheral corneal abrasions heal by the sliding of limbal cells to cover the epithelial defect.1 This response is split into 2 phases: (1) the response of the limbal epithelium, which is the source of the corneal epithelial stem cells, and (2) the response of the conjunctival epithelium itself.
Under normal circumstances, the limbal epithelium acts as a barrier and exerts an inhibitory growth pressure that prevents the migration of conjunctival epithelial cells onto the cornea. Like the rest of the surface of the body, the conjunctiva and the cornea are in a constant state of turnover. Corneal epithelial cells are continuously shed into the tear pool, and they are simultaneously replenished by cells moving centrally from the limbus and anteriorly from the basal layer of the epithelium. Movement from the basal to superficial layers is relatively rapid, requiring 7-10 days; however, movement from the limbus to the center of the cornea is slow and may require months.
This normal physiologic process is exaggerated in the case of a corneal abrasion. During corneal healing of a lesion, corneal epithelial cells become flattened, they spread, and they move across the defect until they cover it completely. Cell proliferation, which is independent of cell migration, begins approximately 24 hours after injury. Stem cells from the limbus also respond by proliferating to give rise to daughter cells called transient amplifying cells. These cells migrate to heal the corneal defect and proliferate to replenish the wounded area.
The observation of limbal pigment migrating onto the clear cornea provided additional evidence of this process. The concept that the limbal cells form a barrier to conjunctival cells was supported further by the observation that rabbit eyes treated for 120 seconds with N -heptanal, which removed the corneal and conjunctival epithelium but left the limbal basal cells intact, healed with the corneal epithelium and had unvascularized corneas. However, when the entire limbal zone was surgically removed along with N -heptanal treatment, corneal vascularization and conjunctivalization was observed.
Demonstration of the centripetal migration of limbal cells (marked by India ink) provided more direct evidence of this concept. These cells migrate in masses as a continuous, coherent sheet, with most cells retaining their positions relative to each other, much like the movement of a herd of cattle.
Rearrangement of intracellular actin filaments plays a role in movement. Cell migration can be inhibited by blocking polymerization of actin, indicating that actin filaments actively participate in the mechanism of cell motion. Some authors believe that conjunctival and limbal epithelial cells may contribute to the regeneration of corneal epithelium. Marked proliferative responses in the conjunctiva after a central corneal epithelium abrasion have been described.
Why the conjunctival epithelium should proliferate in response to a central corneal wound is unknown. One possibility is that the proliferation replenishes the number of goblet cells, which decreases by up to 50% after corneal wounding. However, proliferation occurs at high levels in the bulbar conjunctiva, which contains few if any goblet cells. The apparent decrease in cell number is more likely the result of mucin secretion rather than actual loss of goblet cells. Alternately, conjunctival cells may migrate into the limbus or cornea to help replenish the wound area. No firm data suggest that conjunctival epithelium migrates onto the corneal surface in the presence of intact limbal epithelium. Last, healing of the corneal epithelial wound is not complete until the newly regenerated epithelium has firmly anchored itself to the underlying connective tissue.
Permanent anchoring units are not formed until the wound defect is covered completely. Epithelial cells migrate rapidly and develop strong, permanent adhesions within 1 week when the basement membrane is regularly formed and released during the cell migration process.
Although transient attachments are regularly formed and released during the cell migration process, formation of normal adhesions takes 6 weeks, according to Dua et al.2 Tiny buds of corneal epithelium are present along the contact line between the normal corneal epithelium and the migrating conjunctival epithelium. These buds arise from the corneal epithelium, and normal corneal epithelium appears to replace the conjunctival epithelium by gradually pushing it toward the limbus. The magnitude and extent of both the conjunctival and corneal regenerative responses to a corneal abrasion are correlated with the size of the wound. Large erosions were reported to induce a pronounced response in the rate of epithelial cell migration and mitosis at the limbus.
Insults caused by chemical injuries, Stevens-Johnson syndrome, contact lens–induced keratopathy, and aniridia result in limbal damage. These insults cause delayed healing of the cornea, recurrent epithelial erosions, corneal vascularizations, and conjunctival epithelial ingrowth.
Role of the epithelial defect
A long-standing clinical observation is that corneal abrasions and bacterial corneal infections do not occur in patients with an intact, healthy epithelium. Bacterial keratitis and abrasions develop in 1 of 3 types of patients: (1) those with trauma to the cornea; (2) those with epithelial defects due to intrinsic disease (eg, dry eye, exposure keratitis, neurotrophic keratitis, postinfectious persistent epithelial defects); and (3) those who wear contact lenses, especially extended-wear hydrophilic lenses.
The common feature among the 3 groups is a defect in the corneal epithelium to which the bacteria must adhere to start the infection. Mechanisms underlying the development of epithelial defects in the first 2 groups are self-evident. In the third group, contact lenses may lead to epithelial injury in different ways. The cornea can be injured by insertion or removal of the lens, by trauma from defects in or deposits on the lens, by lens-induced hypoxia, or by chemical toxicity from contact-lens disinfectants.
Defects in the epithelium need not be full thickness. Overnight wearing of soft lenses, which do provide inadequate oxygen transmissibility to prevent hypoxia, causes superficial desquamation of epithelium and increases the propensity for abrasions. Corneal swelling induced by overnight wearing of contact lenses is the most important factor. The cornea normally swells 2-4% during sleep. With a contact lens, overnight swelling is increased to an average of 15%, and gross stromal edema can be present on awakening. In some patients, induced corneal swelling can be sufficient to cause bullae; these can rupture, leading to epithelial defects.